Molecular Oncology
Overview
CELLS store basic and encrypted information to live on the genomic DNA. Cellular behavior is regulated by modification on the genome, so-called epigenome, which determines genetic information to be or not to be utilized in each cell type. Environment could alter epigenome, which might lead to disruption of physiological cellular function and thus cause diseases e.g. cancer, the leading cause of death in our country. Department of Molecular Oncology, formerly 2nd Department of Biochemistry, will elucidate critical epigenomic modifications and their aberrations to cause cancer.
Professor:
Atsushi Kaneda
TEL: +81-43-226-2039
FAX: +81-43-226-2039
e-mail: kaneda●chiba-u.jp
URL: http://www.m.chiba-u.ac.jp/class/moloncol/
※ Please change "●" mark to at-mark if you send emails.

Research & Education
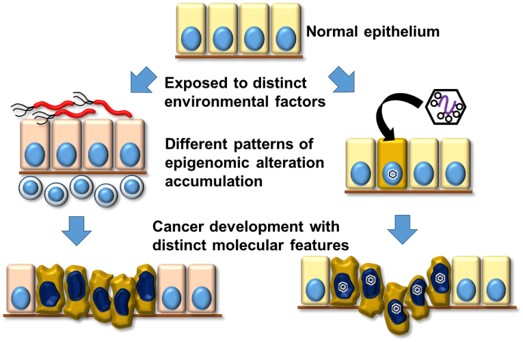
CANCER arises through accumulation of epigenomic and genomic aberrations, and therefore is stratified into several molecular subtypes using comprehensive epigenomic and genomic information1. Environment could alter epigenome as Epstein Barr virus infection induces extensive DNA hypermethylation in gastric cells including critical tumor-related genes, partly due to repression of demethylating enzyme2,3. Epigenomic aberrations accumulated posteriori in apparently normal cells could causally modify tumor risk, so that therapy targeting critical epigenetic aberration would be possible. We will elucidate environmental and molecular causes for such epigenomic aberrations and genesis of tumor in each subtype, which leads to establishment of epigenetic cancer therapy. At School of Medicine, we are responsible for education of metabolic biochemistry. As deficiency of α-ketoglutarate interferes processes of histone and DNA demethylation and thus causes aberrant methylation, teaching the physiological pathway of glycolipid metabolism and the responsible enzymes will help medical students to understand normal and pathological cellular states.
Recent Publications
- Okabe A, Huang KK, Matsusaka K, Fukuyo M, Xing M, Ong X, Hoshii T, Usui G, Seki M, Mano Y, Rahmutulla B, Kanda T, Suzuki T, Rha SY, Ushiku T, Fukayama M, Tan P, Kaneda A. Cross-species chromatin interactions drive transcriptional rewiring in Epstein-Barr virus positive gastric adenocarcinoma. Nat Genet 2020 Sep;52(9):919-930.
- Hata A, Nakajima T, Matsusaka K, Fukuyo M, Nakayama M, Morimoto J, Ito Y, Yamamoto T, Sakairi Y, Rahmutulla B, Ota S, Wada H, Suzuki H, Iwata T, Matsubara H, Ohara O, Yoshino I, Kaneda A. Genetic alterations in squamous cell lung cancer associated with idiopathic pulmonary fibrosis. Int J Cancer 2021 Jun 15;148(12):3008-3018.
- Matsusaka K, Funata S, Fukuyo M, Seto Y, Aburatani H, Fukayama M, Kaneda A. Epstein-Barr virus infection induces genome-wide de novo DNA methylation in non-neoplastic gastric epithelial cells. J Pathol 2017 Aug;242(4):391-399.
- Saju P, Murata-Kamiya N, Hayashi T, Senda Y, Nagase L, Noda S, Matsusaka K, Funata S, Kunita A, Urabe M, Seto Y, Fukayama M, Kaneda A, Hatakeyama M. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by EBV. Nat Microbiol 2016 Mar 14;1:16026.
