About us
Latest research
Yusuke Endo(Lecturer)
Title of the paper
Endo, Y., Hirahara, K., Iinuma, T., Shinoda, K., Tumes, D. J., Asou, H. K., Matsugae, N., Obata-Ninomiya, K., Yamamoto, H., Motohashi, S., Oboki, K., Nakae, S., Saito, H., Okamoto, Y., and Nakayama, T.: The Interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity 42:294-308 (2015)
What's new about the study?
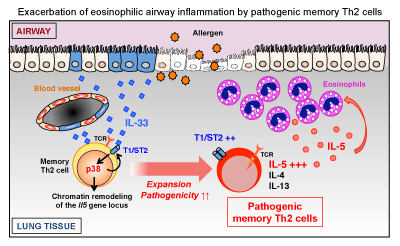
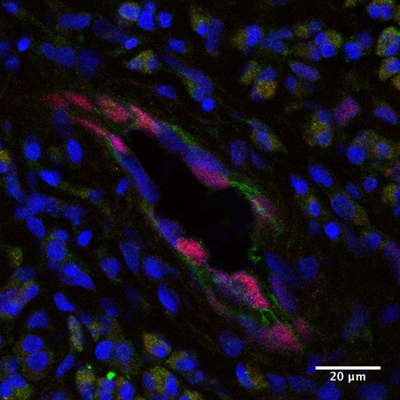
Memory helper T (Th) cells play a central role of chronic inflammatory diseases such as asthma, while functioning as a mainstay of immunological memory. Our team has named a group of memory Th cells that cause diseases as “Pathogenic Memory Th2 Cells”, and we are now working on the clarification of its mechanism. Although pathogenic memory Th2 cells are closely related with chronic allergic diseases, little is known about external signals and internal factors that induce pathogenic memory Th2 cells. In our current study, we found that inflammatory cytokine IL-33 confer memory Th2 cells to activate p38 MAP kinase, which induce memory T helper 2 cell pathogenicity both in mouth models and in samples of patients with chronic sinus infection.
Share your war stories!
The hardest part of the study was the process of discovering that a patient’s sample was a key to success. Although there are similarities between humans and mice, many differences still exist. Often, the same outcome cannot be expected in humans nor can it be expressed with confidence; even for the experiments conducted repeatedly in mice. First, I analyzed memory CD4 T cells taken from peripheral blood of myself or of research team members to discover that there was no reaction to IL-33. I was almost convinced that humans were different from mice. However, thanks to cooperation from an otolaryngologist, I was able to analyze samples of chronic sinus patients. They responded perfectly to IL-33, suggesting IL-33 induces pathogenic memory Th2 cells in humans too. The results of this experiment show the importance of IL-33- pathogenic memory Th2 cells in humans and also its effect on the condition.
What do you expect from the results in the future?
In the past studies, we proposed “Pathogenic memory Th population disease induction model.” The current study provides evidence to support our proposal. It also suggested that IL-33-T1 / ST2- pathogenic memory Th2 cell-induced feedback loop, which exacerbates the condition, leading to chronicity of allergic diseases such as asthma and sinus infection. In other words, the study has great potential for curative development even for intractable chronic allergic diseases by targeting IL-33, and pathogenic memory Th2 cells which express IL-33 receptors, and p38, a molecule that works at downstream of IL-33 and induces pathogenic.
What's next?
The study highlighted that IL-33 stimulates memory Th2 cells functions to induce pathogenic memory Th2 cells, and that a p38MAPK pathway, one of intracellular signal transduction pathways, plays an important role in functional mechanism of IL-33. The next step is to clarify the differences between pathogenic memory Th2 cells and recently identified type 2 natural lymphocyte (ILC2 cells) both at the cell and the genetic level. Furthermore, I will continue to research the roles of cells in the chronicity of allergic diseases. I intend to focus on a feedback mechanism that causes chronicity, or on expressed molecules to see to what extent each cell contributes in pathogenesis and maintenance of individual allergic disease depending on the condition and background of the disease. I believe these steady and persistent efforts are essential to establish a base of disease treatment in the future.